The pharmaceutical industry stands at a crossroads. After decades of remarkable progress, innovation rates are declining while merger and acquisition activity surges; a classic sign that companies are looking externally for growth when internal R&D pipelines fall short. Within this challenge lies an unprecedented opportunity: artificial intelligence is emerging as the catalyst that will usher in a new era of differentiation through innovation.
The innovation-dealmaking cycle
The life sciences industry has long operated in predictable cycles where innovation and consolidation alternate in dominance. When breakthrough discoveries slow, companies turn to acquisitions to fill pipeline gaps and maintain growth trajectories. This pattern has intensified in recent years.
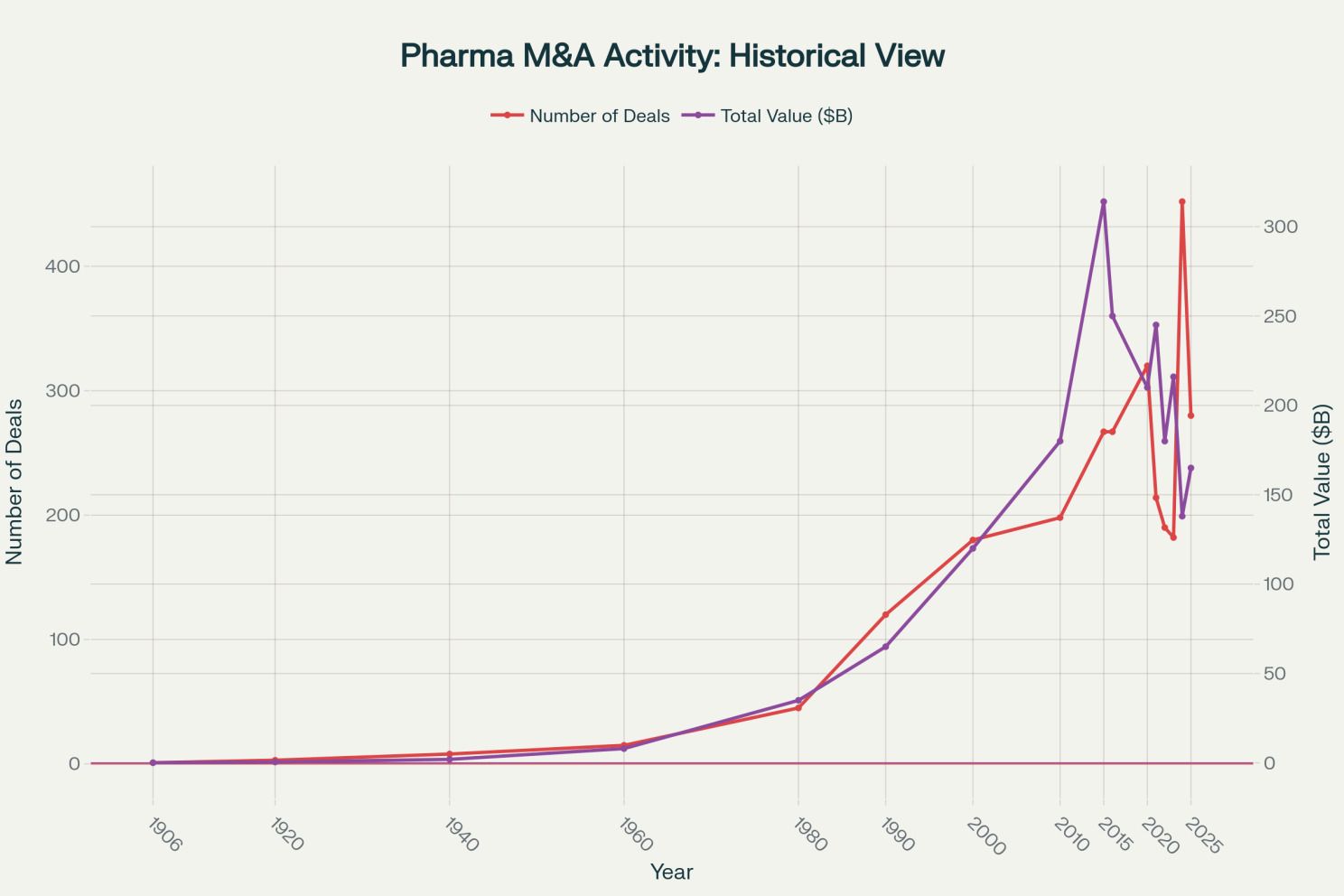
Historical pharmaceutical M&A activity from the early 1900s to today, showing dramatic increase in consolidation activity [1][18]
The historical perspective reveals an acceleration in M&A activity since the 1990s. 2024 saw a record 452 M&A deals worth $138 billion. The industry faces an impending $236 billion patent cliff between 2025 and 2030, with nearly 70 blockbuster drugs losing exclusivity. Major revenue drivers including Merck's Keytruda, Bristol-Myers Squibb's Eliquis, and Johnson & Johnson's Stelara are all approaching patent expiration. This looming cliff is forcing pharmaceutical giants into defensive strategies: cutting costs, acquiring external assets, and consolidating operations rather than investing in breakthrough innovation. [1][2][3]
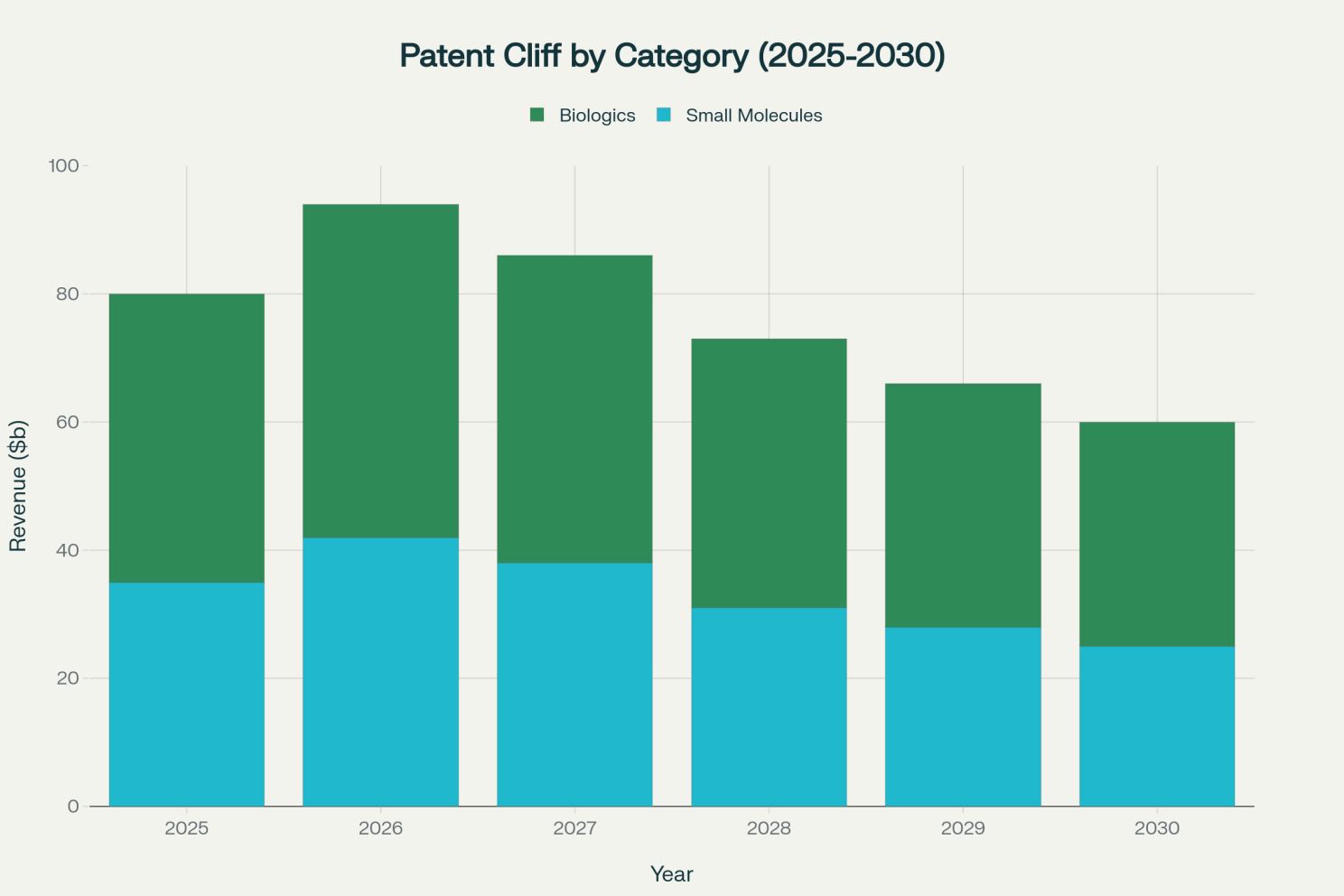
Projected pharmaceutical revenue at risk from patent expiries, highlighting the impact on both small molecules and biologics [2]
The patent expiry data reveals the magnitude of this challenge, with biologics bearing particularly heavy exposure as the industry's shift toward complex therapies reaches maturity. Companies that built their success on biologics innovations now face the same commoditization pressures that small molecule pioneers experienced decades earlier.
The historical arc of pharmaceutical innovation
Understanding today's innovation challenges requires examining how we arrived at this point. The pharmaceutical industry's evolution follows a clear trajectory from abundant opportunities to increasingly scarce breakthroughs. To understand the significance of this evolution, it's essential to recognize what distinguishes different categories of therapeutic products:
Small molecules are traditional chemical drugs with molecular weights typically under 500 daltons. These include familiar medications like aspirin, penicillin, and most oral medications. Small molecules can often be taken orally, are relatively simple to manufacture, and generally have lower development costs.[4][5]
Biologics are large, complex molecules derived from living cells, including proteins, antibodies, and vaccines. These therapeutic products typically have molecular weights of thousands of daltons and require sophisticated manufacturing in living systems. Examples include insulin, monoclonal antibodies like Keytruda, and gene therapies.[5][4]
TIDES (peptides and oligonucleotides) represent an emerging category of therapeutics that fall between small molecules and biologics in size and complexity. This includes therapeutic peptides, RNA-based drugs, and antisense oligonucleotides; molecules that can target previously "undruggable" proteins and pathways.[6]
Gene and cell therapies are the newest category of therapeutics, involving the introduction of genetic material into a patient's cells to treat disease or the use of living cells as therapeutic agents. These represent the most complex and expensive therapeutic modalities.[7]
The industry has evolved through the following general time frames:
The golden age of small molecules (1800s-1960s): The early pharmaceutical era was defined by the discovery of transformative small molecule drugs. Alexander Fleming's accidental discovery of penicillin in 1928 exemplified this period's serendipitous breakthroughs. Insulin extraction, aspirin synthesis, and countless other small molecule innovations emerged from relatively simple laboratory work, creating the foundation of modern medicine. These compounds represented the "low-hanging fruit"; molecules with clear therapeutic effects that could be discovered through traditional chemistry and biological observation.[8][9][10]
The biotech revolution (1980s-2000s): As simple small molecule targets became exhausted, the industry pivoted toward biologics; complex proteins, antibodies, and other large molecules produced by living cells. This shift required entirely new manufacturing capabilities and regulatory frameworks but unlocked treatments for previously untreatable conditions. Monoclonal antibodies, in particular, revolutionized cancer treatment and autoimmune diseases. However, biologics development demanded significantly higher investment, longer timelines, and more sophisticated scientific expertise.[4][5]
The era of complexity (2010s-present): Today's drug development focuses on increasingly sophisticated modalities: gene therapies, cell therapies, antibody-drug conjugates, and oligonucleotides. While these approaches offer unprecedented precision, they also represent the industry's most challenging frontier. Development costs have soared, regulatory pathways remain uncertain, and manufacturing complexity continues to grow. The 2024 approval of nine gene and cell therapy products by the FDA represents a landmark year for this emerging category.[7][12][13][4]
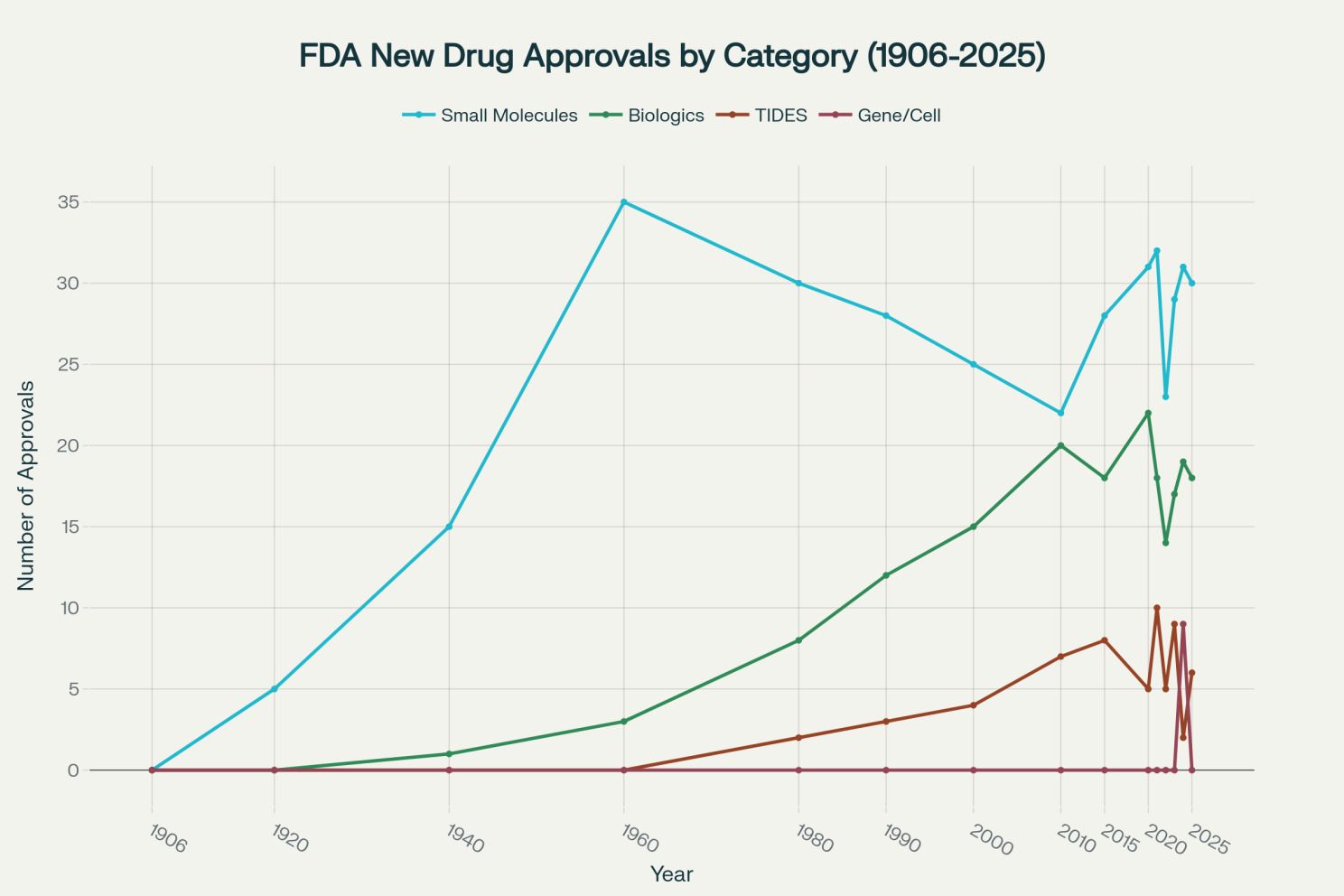
FDA new drug approvals by therapeutic category from 1906-2025, showing the evolution from small molecules to complex therapies [4][8][11]
The FDA approval data illustrates this evolution clearly. While total approvals have grown modestly since the mid-20th century, the composition has shifted. Small molecules, once dominant, now compete with an expanding array of complex modalities. The 2024 data shows small molecules comprising 52.5% of approvals, biologics 32.2%, and gene/cell therapies representing a significant 15.3% of new approvals.[7]
The productivity crisis
Perhaps most concerning is the dramatic decline in innovation productivity despite massive increases in R&D investment.
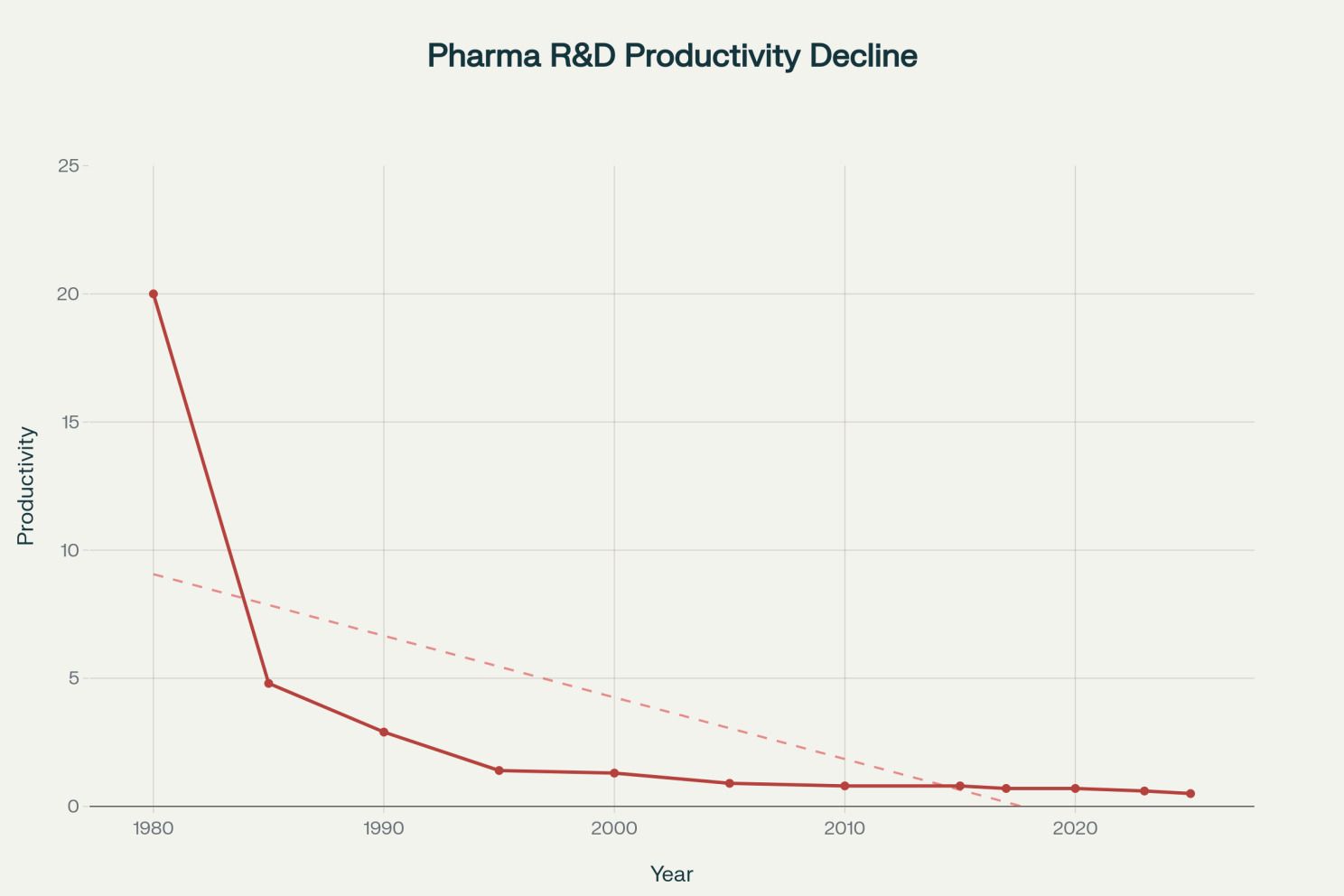
Declining pharmaceutical R&D productivity showing a 40-fold decrease from 1980 to 2025 [10][12][13]
This productivity crisis represents one of the pharmaceutical industry's greatest challenges. From 1980 to 2025, R&D spending increased from $2 billion to over $105 billion annually, yet the number of drug approvals has grown only modestly. The result is a 40-fold decrease in productivity, measured as approvals per billion dollars invested. This decline reflects the "fishing out" effect; the easiest therapeutic targets have been addressed, leaving increasingly complex diseases that require more sophisticated and expensive approaches.[13][14][15][16]
AI as the innovation catalyst
Artificial intelligence represents more than just another tool for pharmaceutical R&D; it's a fundamental shift in how drugs are discovered, developed, and delivered to patients. The technology is already demonstrating remarkable results across multiple dimensions of pharmaceutical development.
Accelerating discovery timelines
Traditional drug discovery requires 10-15 years and over $2 billion in investment. AI is compressing these timelines dramatically. Insilico Medicine identified a novel drug target and designed a molecule for idiopathic pulmonary fibrosis that reached Phase II clinical trials in just 18 months; a timeline that would typically require 5-7 years. Similarly, Exscientia brought an AI-designed molecule for obsessive-compulsive disorder to Phase I trials in just 12 months, demonstrating AI's ability to navigate the entire discovery-to-clinical pathway.[5][6][14][16][17]
Improving success rates
Perhaps most significantly, AI-discovered drugs are showing superior clinical performance. Molecules identified through AI exhibit 80-90% success rates in Phase I clinical trials, compared to 40-65% for traditionally discovered drugs. This improvement suggests AI's particular strength in identifying drug-like properties and optimizing molecules for safety; historically major sources of clinical failure.[13][16][11]
Enabling precision medicine
AI's data processing capabilities unlock personalized treatment approaches that were previously impossible. Machine learning models can analyze genomic data, electronic health records, and clinical outcomes to predict individual patient responses. This precision reduces trial-and-error prescribing and enables development of targeted therapies for smaller patient populations that were previously commercially unviable.[10][11]
Transforming R&D economics
The productivity implications are profound. AI could potentially double overall R&D productivity by increasing the probability of clinical success from the historical 5-10% to 9-18%. This improvement would enable companies to either reduce costs for the same output or increase the number of new drugs brought to market within existing resource constraints.[11][13]
Real-world impact across applications
Drug target identification: AI platforms analyze genomic and clinical data to identify novel disease targets in weeks rather than months[8]
Molecular design: Generative AI creates novel molecular structures with desired properties before any laboratory synthesis[3][8]
Clinical trial optimization: AI-enabled trial processes demonstrate significant cost savings and timeline reductions[8]
Drug repurposing: AI identifies new therapeutic applications for existing drugs, dramatically reducing development risk and cost[11]
Manufacturing optimization: Predictive analytics improve quality control and reduce manufacturing inefficiencies[6]
Looking ahead: differentiation through strategic AI adoption
The pharmaceutical companies that will thrive in the coming decade won't simply use AI to do traditional R&D faster; they'll leverage AI to pursue entirely new approaches to innovation and differentiation. The distinction between efficiency-focused AI adoption and innovation-driven AI strategy will separate industry leaders from followers.
The efficiency trap
Many pharmaceutical companies are implementing AI primarily as a cost-reduction tool, using it to automate existing processes or accelerate traditional discovery methods. While this approach delivers near-term benefits, it fails to capture AI's transformative potential. Companies focused solely on efficiency improvements risk commoditizing their R&D capabilities and competing primarily on operational excellence rather than therapeutic innovation.
The differentiation opportunity
Leading companies are using AI to enable fundamentally new types of innovation:
Targeting "undruggable" proteins: AI enables drug design for protein targets previously considered impossible to address, opening entirely new therapeutic areas[3]
Multi-target drug design: Machine learning can optimize molecules for complex interactions across multiple biological targets simultaneously[8]
Predictive patient stratification: AI identifies patient subgroups likely to respond to specific treatments before clinical trials begin, enabling more focused development strategies[11]
Dynamic formulation optimization: AI continuously optimizes drug formulations based on real-world patient data and manufacturing feedback[9]
Building AI-driven innovation capabilities
Success requires more than technology implementation; it demands organizational transformation:
Leadership and vision: Companies need executives who understand AI's strategic potential beyond cost savings. This means investing in AI literacy at the C-suite level and developing long-term AI strategies aligned with therapeutic goals rather than purely operational metrics.
Cross-functional integration: AI works best when integrated across disciplines. The most successful companies are breaking down silos between computational scientists, medicinal chemists, clinicians, and regulatory experts. This integration enables AI insights to inform decisions throughout the drug development process.
Data strategy and infrastructure: AI's effectiveness depends entirely on data quality and accessibility. Leading companies are investing heavily in data integration platforms that combine internal R&D data with external genomic databases, clinical outcomes, and real-world evidence sources.
Talent and culture: AI-driven innovation requires new types of expertise. Companies need computational biologists, machine learning engineers, and data scientists who understand both AI technologies and pharmaceutical science. Equally important is fostering a culture that embraces data-driven decision-making and tolerates the experimentation inherent in AI development.
Strategic recommendations
For pharmaceutical companies seeking to leverage AI as a competitive differentiator:
Identify white space opportunities: Use AI to pursue therapeutic areas or patient populations that competitors have abandoned due to traditional development challenges. AI's ability to work with limited datasets makes previously unviable indications potentially viable.
Invest in proprietary AI platforms: Rather than relying solely on vendor solutions, develop internal AI capabilities that can create sustained competitive advantages. The companies building proprietary AI platforms today will own unique datasets and algorithms that competitors cannot easily replicate.
Focus on patient outcomes, not just efficiency: Measure AI success by improvements in patient outcomes and therapeutic differentiation rather than purely operational metrics. This patient-centric approach drives innovation that creates lasting competitive moats.
Build ecosystem partnerships: No single company can master all aspects of AI-driven drug development. Strategic partnerships with AI companies, academic institutions, and technology providers accelerate capabilities development while sharing risks and costs.
The pharmaceutical industry's innovation slowdown is not inevitable; it's a symptom of exhausting traditional approaches. AI offers a path forward, but only for companies willing to embrace it as a transformative force rather than merely another efficiency tool. The organizations that recognize AI's potential to reignite genuine innovation, rather than simply accelerate or add efficiencies to existing processes, will define the next era of pharmaceutical leadership.
The choice is clear: use AI to compete on the same old battlefield more efficiently or use it to create entirely new battlefields where differentiated innovation drives sustainable competitive advantage. The companies that choose the latter will not only survive patent cliffs; they'll use it as a launching pad for the next wave of breakthrough therapies.
References
DealForma. "Global Healthcare and Life Sciences M&A - 2024 Review." DealForma, 7 Feb. 2025, https://dealforma.com/global-healthcare-and-life-sciences-ma-2024-review/.
"Pharmaceutical Industry Braces for $236 Billion Patent Cliff by 2030." Medpath, 6 Mar. 2025, https://trial.medpath.com/news/bdeaa1ba345678a3/pharmaceutical-industry-braces-for-236-billion-patent-cliff-by-2030-strategic-responses-from-major-players.
"10 Best-Selling Drugs of 2024 Rake in Billions Amid Exclusivity Threats." BioSpace, 4 Mar. 2025, https://www.biospace.com/business/10-best-selling-drugs-of-2024-rake-in-billions-amid-exclusivity-threats.
"How AI Is Reshaping Pharma: Use Cases, Challenges." Whatfix, 19 May 2025, https://whatfix.com/blog/ai-in-pharma/.
"The Food and Drug Administration: the Continued History of Drug Advertising." Weill Cornell Medicine Library, 31 Dec. 2024, https://library.weill.cornell.edu/about-us/snake oil -social media-drug-advertising-your-health/food-and-drug-administration-continued.
"Biologics vs Small Molecules: A New Era in Drug Development." Synerg BioPharma, 20 Jan. 2025, https://synergbiopharma.com/biologics-vs-small-molecules/.
"AI in Life Sciences: Top 5 Use Cases in 2025." Netguru, 8 Sep. 2025, https://www.netguru.com/blog/ai-use-cases-in-life-sciences.
"An Analysis of FDA Drug Approvals from the Perspective of Molecules." PMC, 24 Jan. 2024, https://pmc.ncbi.nlm.nih.gov/articles/PMC10856271/.
"A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis." Nature Medicine, 3 Jun. 2025, https://www.nature.com/articles/s41591-025-03743-2.
"Exscientia's First AI-Designed Drug Enters Phase I to Treat OCD." Labiotech, 23 Jun. 2022, https://www.labiotech.eu/trends-news/exscientia-ocd-ai-sumitomo/.
"AI-discovered drugs achieved higher success rate than those by humans – study." HT World, 9 May 2024, https://www.htworld.co.uk/news/ai/ai-discovered-drugs-achieved-higher-success-rate-than-those-by-humans-study/.
"Incremental decline in R&D efficiency despite transient improvements." PubMed, 22 Nov. 2024, https://pubmed.ncbi.nlm.nih.gov/39241979/.
"Harnessing Artificial Intelligence in Drug Discovery and Development." ACCC Cancer Center, 19 Dec. 2024, https://www.accc-cancer.org/acccbuzz/blog-post-template/accc-buzz/2024/12/20/harnessing-artificial-intelligence-in-drug-discovery-and-development.
"AI-Discovered Drug INS018_055 Enters Phase 2 Trials for Idiopathic Pulmonary Fibrosis." Medpath, 24 Nov. 2024, https://trial.medpath.com/news/06a7daaa6aa5519b/world-s-1st-ai-made-lung-disease-drug-gets-phase-2-trial-in-us-china.
"8 Applications of Machine Learning in The Pharmaceutical Industry." Drug Patent Watch, 27 Aug. 2025, https://www.drugpatentwatch.com/blog/8-applications-machine-learning-pharmaceutical-industry/.
"AI in Pharma: Top 9 Use Cases You Should Know." Litslink, 27 Mar. 2025, https://litslink.com/blog/use-cases-of-ai-in-pharma-how-to-leverage-it.
"A year in pharmacology: new drugs approved by the US Food and Drug Administration in 2024." PMC, 30 Mar. 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11985671/.
"Mergers and Acquisitions (M&As) in Pharmaceutical Markets." U.S. Department of Health and Human Services, 7 Jan. 2025, https://aspe.hhs.gov/sites/default/files/documents/ec5de77c72cff3abf802b5e9c6cc8ae4/aspe-pharma-ma-report.pdf.

